- 2255 - The history of the atom continues. It starts in 1803 with molecules, then atoms, then atomic nuclei, then sub-nuclear particles, then antimatter and the possibility of an anti-Universe. The study of the ever smaller has taken us to the ever larger. And, the history of the atom continues.
-
-
-
---------------------------- - 2255 - History of the atom
-
- In 1803 John Dalton wrote down the first atomic theory. Each element is made up of extremely minute particles, atoms, and different elements are made up of atoms of different weights.
-
- In 1811 Amedeo Avogadro determined that a gas under the same pressure and temperature always contained the same number of molecules. A liter of gas contains 27,000,000,000,000,000,000,000 molecules. This gave us the first quantitative size of an atom.
-
- In 1827 Robert Brown observed pollen randomly moving around in a drop of liquid. It became known as Brownian Motion and remained an unexplained phenomena until 1905.
-
- In 1869 Dimitri Mendeleev developed the periodic table of elements with atomic weights grouped together where elements were having similar chemical behaviors.
-
- In 1905 Albert Einstein wrote a paper with a quantitative theory of how Brownian motion was caused by vibrating atoms. He developed statistical mathematics that showed that the atoms bouncing off the pollen seeds would cause the zig zag motion. These calculations were the first derivation of the size of atoms and their random motion as a function of temperature. A molecule was calculated to be 0.00000001 centimeters diameter.(10^-8 centimeters)
-
- Gas pressure, such as a car tire at 45 pounds per square inch, is actually a measure of the amount of gas molecule collisions against the walls of the tire. At normal temperatures a molecule of oxygen is traveling at 400 meters per second, 895 miles per hour. But a molecule traveling fast does not mean it travels far.
-
- At normal atmospheric pressure the molecule only gets about .00001 centimeters (10^-5) before it collides with another molecule. If a molecule travels 1 centimeter it has had 100,000 collisions in its zig zag path. This is the Brownian motion that was first observed in 1827. The molecule only got .003 centimeters from its original position having experienced 4,000,000,000 collisions per second.
-
- In 1833 Michael Faraday proposed that atoms have elementary parts that behave like “atoms of electricity“. In 1874 these elementary particles were named electrons. In 1869 these electrons were observed in gas filled tubes as cathode rays. In 1897 science finally agreed that the electron was real largely due to J.J. Thomson’s cathode ray tube experiments.
-
- In 1908 to 1913 Millikan preformed his famous oil drop experiments to measure the mass and the charge of an electron. Inside a glass enclosure oil drops were suspended by giving them an electric charge and putting a plate of positive voltage at the top of the jar. As the drops fell through the jar the voltage was adjusted to just stop their fall. When this force of electric charge just balanced the force of gravity the ratio of the two forces could be calculated. The ratio was calculated to be 1800 to 1 between the hydrogen atom and the electron. This was a very delicate experiment requiring lots of patience.
-
- The mass of an electron is .000,000,000,000,000,000,000,000,098 grams. (10^-27). Its electric charge is .0000000048 electro static units. (4.8^10^-10). To illustrate these numbers, the number of electrons that flow through a 100 watt light bulb every second is equal to the number of cubic centimeters (a sugar cube of water) that has flowed down the Russian River from the year 1200 to today.(805 years).
-
- In 1911 the New Zealand physicist, Ernst Rutherford, developed the proton nucleus, electron orbiting model of the atom.
-
- In 1895 X-rays were discovered by Marie Sklodoska Curie. By 1909 Rutherford was experimenting with alpha particles, which were actually protons emitted from a radioactive substance. The particles traveled at 30,000,000 meters/sec, 18,600 miles per second, or about 10% the speed of light.
-
- Rutherford aimed these particles at a thin leaf of gold. The results were totally unexpected. Only one proton in 8,000 was deflected more than 90 degrees. The rest passed right through the metallic leaf. This meant that the gold atoms were mostly empty space with only a small nucleus.
-
- Rutherford calculated that the nucleus was .000000000001 (10^-12) centimeters diameter. To illustrate this number, if the atom was enlarged to a sphere one mile in diameter, the nucleus would be the size of a tennis ball in the center.
-
- Electromagnetic theory at Rutherford’s time was that a charged particle (an electron) under constant acceleration (in circular orbit) should continuously radiate energy. If it did that it would lose energy and spiral into the nucleus. Atoms could not exist?
-
- In 1900 Max Planck and in 1905 Albert Einstein developed the theory of a quantum of energy. That the amount of energy depended on the frequency of the radiation. Red light has 4.3 10^14 wave cycles per second. Violet light has higher energy with a frequency of 7.5 10^14 wave cycles per second.
-
- The amount of energy is some constant times the wave’s frequency. The constant became known as Planck’s constant and was calculated to be 6.610 10^-27 erg * seconds. ( or, 6.625 * 10^-34 kg*m^2 / sec.). Energy is released in discrete packets. It is not continuous radiation as first thought. Einstein gave the name photons to these energy packets, or quantum’s.
-
- In 1912 Niels Bohr explained the stability of atoms by introducing electron shells that orbit the nucleus at specific energy levels. As long as the electron stays at one of these energy levels it radiates no energy. The atom emits, or absorbs, a photon of energy only when an electron jumps to a lower or a higher energy level. The frequency of the photon wave emitted is equal to the difference in the energy levels divided by Planck’s constant.
-
- In 1916 Arnold Sommerfield extended Bohr’s model of the atom to include elliptical orbits for the electrons and Einstein’s relativistic equations for near light velocities.
-
- In 1925 the model was further extended to account for the electron spinning, and as a spinning electric charge behaving like a little magnet. This means that when an electron is in a magnetic field its spin axis will orient itself in the direction of the magnetic field.
-
- Electrons always spin at the same velocity equal to Planck’s constant / 2 pi. (6.28 radians per cycle). The spin can be clockwise or counter-clockwise. These concepts allowed Pauli to define the inner most orbit to contain no more than 2 electrons. The next energy shell could contain no more that 8 electrons and the third energy shell could contain no more than 18 electrons.
-
- When electron shells are full the elements are the noble gases (helium, neon, argon).
-
- When electron shells are full and have one extra electron in the outer shell the elements are alkaline. (lithium, sodium, potassium, rubidium).
-
- When electron shells are nearly full and need only one more electron to become full these elements are halogens. (fluorine, chlorine, bromine, iodine)
-
- After 1925 the history of the atom got a major jolt. The field of physics was turned upside down with the discovery of quantum mechanics.
-
- With the theory of quantum of energy, the photon, the science of Quantum Mechanics was born. De Broglie developed theories of wave mechanics to explain quantum energy behavior. He introduced the concept of particle duality, a photon could be both a point particle and a wave. A photon could spread itself through space and display the interference pattern of a wave. Or, its spreading could stop and it could condense and display itself as a particle.
-
- Heisenberg came up with another approach to explain quantum mechanics using matrix mathematics. In 1927 Heisenberg presented his Uncertainty Principle. His theory concludes that you can know the position of a particle or the velocity of a particle but there is no way to know both position and velocity with certainty. The certainty of position times the certainty of velocity is always equal to a constant.
-
- The constant is Planck’s constant divided by the mass of the particle. The Energy is Planck’s Constant times the frequency of the wave.
-
- The Uncertainty Principle has since been extended to time and energy and to electrical intensity and magnetic intensity. This uncertainty applies to everything, but since Planck’s constant (a very small number)/ mass becomes so negligibly small for larger masses we really do not take it into account until we are dealing with atomic size particles.
-
- However, the uncertainty is always there. Whatever we observe is always a matter of statistics. We are always balancing our knowledge versus our ignorance. The more we know about space (position) the less we know about velocity (motion). The more we know about time the less we know about energy. The magnitudes of these attributes do not have exact values , they have probability values.
-
- Electromagnetic phenomena are based on the electrons and protons (nucleus of the atom). Radioactivity, gamma rays, are based on inside the nucleus. Isotopes are elements having the same number of electrons and protons but have different weights of the nucleus due to neutrons.
-
- The additional neutrons inside the nucleus tend to be unstable, therefore the heavier isotopes tend to be unstable and radioactive. They tend to periodically emit alpha and beta particles, protons and electrons, at known probabilities.
-
- These probabilities are described as the “half-life“, the time in which half of a quantity of that substance will have disentangled. To illustrate: the half-life of thorium is 14,000,000,000 years. Radium is 1,590 years. Thorium C is .00000001 seconds. Radium is 16,000 years. Radon is 4 days.
-
- In addition to the alpha and beta particles, gamma rays are emitted from the radioactive nucleus. They are emitted when the nucleus passes from one quantum state to another which has a lower energy level. A nuclear force called the Strong Force is needed to keep two protons bound in a nucleus, into a sphere .0000000000001 centimeters in diameter. (10^-13).
-
-
- The electrostatic force between two positively charged protons is enormous. To illustrate: If you had a gram of protons in San Francisco and another gram of protons on the opposite side of the Earth, the two charges would still have a repulsive force equal to 28 tons of weight.
-
- The strong force operates over a very short range however. When the 2 protons are more than .0000000000004 centimeters apart (4 10^-13) the strong nuclear force no longer prevails over the electrostatic force.
-
- The concept of energy shells exists for the nucleus itself, very similar to the electron shells that structure the atom. Nuclei with 2,8,20,28,50,82,126 protons or neutrons are exceptionally stable. Which means the nuclear shells are full with 2,6,12,8,22,32,44 protons or neutrons.
-
- In 1934 Enrico Fermi proposed that a neutron could transform into a proton and an electron. A free neutron will live only 12 minutes before going through this transformation. The binding force that keeps neutrons and protons together in the nucleus is the Weak Force and it is carried by a particle called the meson. We cannot see these particles. We have to measure their energies to identify them.
-
- The eye cannot distinguish a particle smaller than .0007 centimeters. With a microscope we can not distinguish a particle smaller than .00005 centimeters. This is still 5,000 times the diameter of an atom and 500,000,000 the diameter of the nucleus.
-
- We need to use E = mc^2 and observe the energies of these particles rather than their mass. Energy is measured in Electron-volts. An electron is 500,000 electron-Volts, or eV. A proton is 1,000,000,000 eV. The nucleus of deuterium is 2,000,000,000 eV. The helium nucleus is 4,000,000,000 eV. These are their energies at rest. On top of this you have to add the kinetic energy of the particles in motion.
-
- Using particle accelerators to attain these energies scientists are able to see the nuclear forces (particles) that bind neutrons to neutrons, neutrons to protons, and protons to protons inside the atomic nucleus. The heavier force particles, or mesons, are called the pi meson, or pion. It has a mass 270 times that of the electron. The mu-meson, or muon, has a mass 205 times that of an electron. Both decay in .000000025 seconds (2,5 10^-8) into a muon and a neutrino.
-
- The Earth has its own particle accelerators in cosmic rays that enter the upper atmosphere from intergalactic space. These are charged particles, mostly protons, traveling at near light speeds. They contain even higher energies than we can attain in our Earth bound man-made accelerators.
-
- To illustrate: here is one result from these studies: Deuterium has a nucleus of one proton and one neutron. The mass of hydrogen (one proton and one electron) = 1.0081. The mass of a neutron is 1.0090. Therefore the mass of deuterium = 1.0081 + 1.0090 = 2.0171. However, when we measure the mass of deuterium we get 2.0147, a difference of 0.0024 units of atomic weight. According to E=mc^2 this difference in mass is 2,230,000 electron volts of energy.
-
- The proton and neutron in the nucleus of deuterium are bound together with an energy 250,000 times greater than the energy which hold two atoms of a molecule of hydrogen together. When a proton captures a neutron to form a deuterium nucleus the emitted gamma ray radiation is exactly 2,230,000 electron volts.
-
- Nuclei experience 2 types of reactions. Fusion where light nuclei fuse to create heavier nuclei and fission where heavier nuclei divide to create lighter nuclei. To get a fusion reaction takes an enormous amount of energy. At 100,000,000 degrees temperature, with a pressure of 15,000,000 atmospheres, with electrons traveling 90,000 miles per second, and deuterium nuclei traveling 1,500 miles per second a nuclear fusion reaction can create 100,000,000 kilowatts of energy. To sustain this reaction the temperature would need to be further increased to 300,000,000 degrees.
-
- The fusion reaction in the center of our Sun fuses 564,000,000 tons of hydrogen into 560,000,000 tons of helium every second. 4,000,000 tons of matter is converted into energy every second. Gamma ray radiation is emitted from the core and heads for the surface. Gamma rays making their way to the surface lose energy and eventually leave the surface as visible light.
-
- In 1942 the first fission reactor became operational in Chicago. It started at ½ watt of power and after a few months got to 18,000,000 watts, or 18,000 kilowatts.
-
- Understanding fusion and fission allowed us to learn more about inside the atom. The photon, carrier of the electromagnetic force, has zero rest mass. It cannot exist at rest but always moves at 186,000 miles per second.
-
- “Spin” along with mass and electric charge is another intrinsic property of particles. A photon has a spin of 1. An electron has a spin of ½. All particles with spin of ½ obey Pauli’s Exclusion Principle, which states only one electron can exist in each orbit. These particles with spins of ½ are called fermions because they also obey the statistics of Fermi-Dirac.
-
- In contrast, all particles that have a spin of 0 or 1 do not obey Pauli’s Exclusion Principle. Photons with spin of 1 can occupy the same space as another photon. Photons and spin 1 particles are called bosons because the follow the statistics of Bose-Einstein.
-
- Protons and neutrons are held together by pi-mesons, or pions. At energies above 135,000,000 electron volts pions materialize as real particles.
-
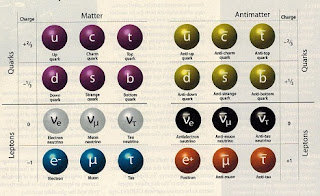
- Particle physics has identified 12 fundamental particles and 12 equivalent anti-particles. These all obey the laws of physics, the conversation of energy, the conservations of angular and linear momentum, the conversation of electric charge.
-
- The strong force interactions are the fastest possible force. The absorption and emission of a pi-meson occurs in 10^-23 seconds. This is the time it takes light to travel the diameter of an atomic nucleus, .0000000000003 centimeters (3*19^-13).
-
- The electromagnetic force interactions using photons is about 140 times slower. The weak interaction force is about 10^-9 seconds. Comparing it to the speed of the strong force is like comparing 1 second to 1,000,000 years.
-
- In 1950 the study of cosmic rays found even more particles at even higher mass and higher energies. Making sense of all these fundamental particles becomes a significant challenge. The one hope in physics is that the laws of symmetry and conversation hold. One law of symmetry is the conservation of strangness, or the conservation of isotropic spin. Another is the conservation of parity, that there is no distinction between a real event and its mirror image.
-
- In 1957 evidence first materialized that there are three different types of neutrinos. By this time we had 30 elementary particles identified. Matter has existed for billions of years so we can conclude that the proton, electrons and neutrons are stable particles. There must be laws of conservation and symmetry that have held these particles together.
-
- However, one law of symmetry seems to be broken between matter and anti-matter. Both seem to respond to gravity as an attractive force and there is no evidence of anti-gravity as a repulsive force. If anti-gravity does exist maybe there is another Universe somewhere made up of entirely antimatter.
-
- Our history of the atom started with molecules, then atoms, then atomic nuclei, then sub-nuclear particles, then antimatter and the possibility of an anti-Universe. The study of the ever smaller has taken us to the ever larger. And, the history of the atom continues.
-
- February 3, 2019. A superbowl of knowledge 610 and 611
----------------------------------------------------------------------------------------
----- Comments appreciated and Pass it on to whomever is interested. ----
--- Some reviews are at: -------------- http://jdetrick.blogspot.com -----
-- email feedback, corrections, request for copies or Index of all reviews
--- to: ------ jamesdetrick@comcast.net ------ “Jim Detrick” -----------
- https://plus.google.com/u/0/ -- www.facebook.com -- www.twitter.com
--------------------- Sunday, February 3, 2019 -------------------------
-----------------------------------------------------------------------------------------
No comments:
Post a Comment